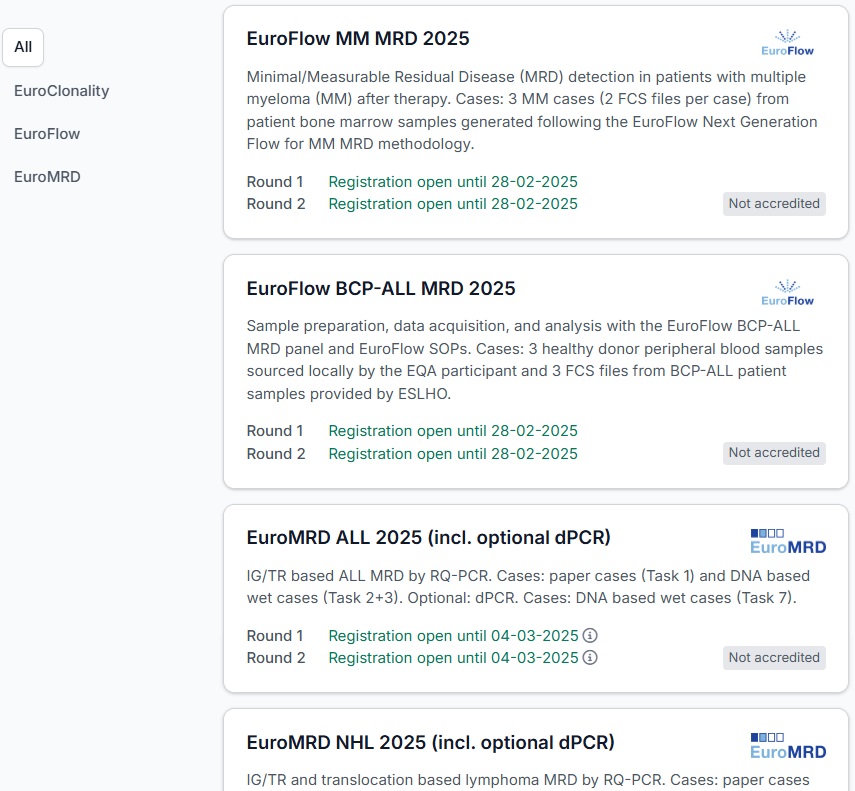
EuroMRD ALL 2025 (incl. optional dPCR)
EuroMRD ALL EQA scheme 2025
Task 1-3: IG/TR-based RQ-PCR
Within these tasks, quality assessment for IG/TR based minimal residual disease (MRD) RQ-PCR for acute lymphoblastic leukemia (ALL) is performed: Task 1 deals with correct interpretation of IG/TR-RQ-PCR based on real-time quantitative PCR data of 10 patient cases presented as electronic files. Task 2 consists of IG/TR target detection and sequence analysis in a diagnostic sample and subsequent quantification of MRD in follow-up (FU) samples. Task 3 includes analysis of provided IG/TR sequences and RQ-PCR based MRD quantification in FU samples.
Always combined with the mandatory Task 1, Task 2 is issued for the spring and Task 3 for the autumn EQA round.
Optional: Task 7: dPCR for MRD quantification in ALL (IG/TR)
The use of dPCR for the quantification of MRD in lymphoid malignancies has seen a marked increase. Within EuroMRD, dPCR ring trials (pilot) have been conducted and utilized for the standardization and establishment of guidelines for dPCR-based MRD quantification in ALL. IG/TR dPCR is performed on the same DNA provided for Tasks 2 or 3. dPCR analysis should be conducted in accordance with the instructions provided by the organizers.
Please indicate whether or not you join the dPCR part/Task 7 in the "Note for ESLHO" field during registration (after you select your schemes and click "Finish registration"). This is important because you will receive a separate set of samples for the dPCR task. Thank you!
Performance evaluation and report
Certificates of participation and performance in EQA rounds will be distributed after the EuroMRD meeting of the same calendar year. Performance criteria of the ALL scheme: a certificate will be provided if at least 75% of points have been received (spring round: Tasks 1 & 2; Autumn round: Tasks 1 & 3), otherwise only a certificate of attendance will be provided for the respective tasks.
EQA Educational meeting
During the ESLHO week in November, the annual EuroMRD meeting is organized. At this meeting, the results of all EuroMRD EQA schemes are shown (anonymized) and discussed with all EuroMRD participants, in small discussion groups as well as during plenary sessions.
Registration and participation fee
The EuroMRD ALL EQA scheme is only available for participants of the EuroMRD Consortium that are part of the ALL section. The participation fee is € 250. In addition, the EuroMRD annual fee is charged once to every EuroMRD participant.
Organization
The ALL EQA scheme is organized by ESLHO (EQA Program Coordinator: Prof. Dr. Jacques J.M. van Dongen) in collaboration with the EuroMRD EQA Committee (ALL scheme lead: Prof. Dr. Monika Brüggemann; dPCR lead Dr. Daniela Drandi). The members of the EuroMRD EQA Committee are participants of the EuroMRD Consortium. The EuroMRD subject-matter experts provide support for:
Pre-round: Case selection, determination of consensus results
Post-round: Data analysis, performance evaluation, reporting
Details on the task division can be found below.
Task division
Name | Organization/Institute | Tasks |
|---|---|---|
Prof. Dr. Monika Brüggemann | UKSH, Kiel, DE | Lead ALL scheme |
Dr. Heiko Trautmann | UKSH, Kiel, DE | Organization, results forms, instructions, data analysis, reporting |
Dr. Jan Kässens | UKSH, Kiel, DE | Results forms, data analysis |
Dr. Britta Kehden | UKSH, Kiel, DE | Data analysis, reporting |
Dr. Daniela Drandi | University of Torino, Torino, IT | Coordination dPCR pilot |
Round 1 (spring): ROT/UTR Round 2 (autumn): KIEM | Case selection, providing DNA, Task 1 preparation | |
Dr. Julia Alten, Dr. Eva Froňková, Dr. Mirkka Montonen, Dr. Jeremy Hancock, Dr. Heiko Trautmann | EuroMRD EQA Committee members | Reference data/consensus results |